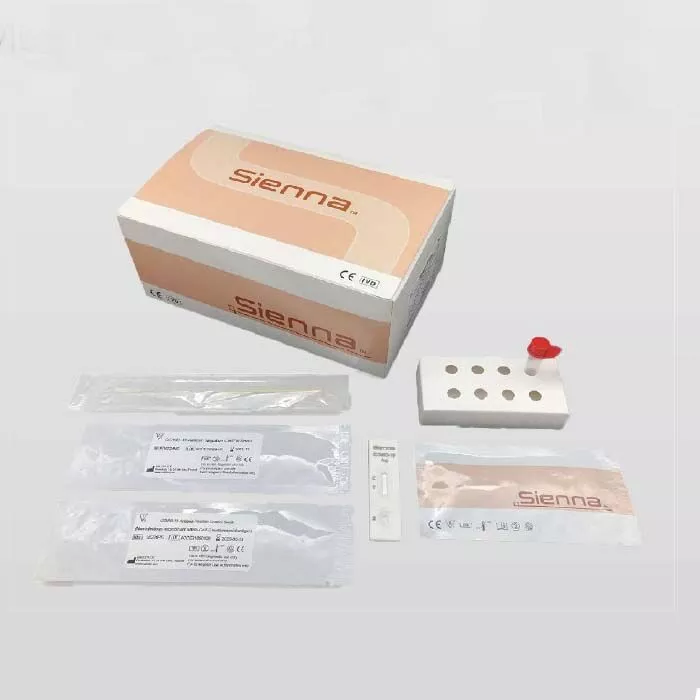
Description
Sienna Rapid Antigen Test Specifications:
| Product Name | Sienna Rapid Antigen Test |
| EUA Number | EUA210062 |
| Result Time: | 10 minutes |
| Authorized Use: | For professional in vitro diagnostic use only |
| LOD | 1.25 X 10 3 TCID 50/ml |
| Individual Buffer Vials | Helping multiple operators at the same time |
| Specimens Obtained Through | Direct nasopharyngeal swab |
| Each Kit Includes |
|
| Positive Predictive Value | 100% with CT Value – 21-33 |
| Negative Predictive Value |
98.9% with CT Value – 21-33 |
| Manufacturer |
Salofa Oy |
| Manufacturing Country | Finland |
Applications:
Quickly screen people for COVID-19 at CLIA-certified labs, places of work, universities and schools, airports and train stations, stadiums, hotels, cruise ships, and tours.
As a reminder: These tests are for professional in vitro diagnostic use only.








Reviews
There are no reviews yet.